P-v Phase Diagram For Water [diagram] Stroke Volume Diagram
Solved 3. (a) draw p-t and p-v phase diagrams, identify Solved a) draw a typical p-v phase diagram, and on this 2.3 phase diagrams – introduction to engineering thermodynamics
PV Diagram for Pure Systems | PNG 520: Phase Behavior of Natural Gas
Solution: p v diagram of water Phase ice water diagram why slide above time do Phase changes
Pv diagram for water
Pvt phase diagramOn a t-v diagram, sketch an isobar through the vapor, vapor + liquid P-v diagram waterDiagram phase pure envelope substance pv point dew bubble critical gas liquid line showing region two vapor natural behavior systems.
Pv phase isotherms constant pressure engineering below pageindexPhase diagram of water (h2o) 16+ pv diagram of water10.4: phase diagrams.

Pv diagram of water
2.3 phase diagrams – introduction to engineering thermodynamicsSolved 5. sketch the p-v diagram for water in a process at Solved consider transcribed textPv diagram for water.
2.3 phase diagrams – introduction to engineering thermodynamicsTemperature phase physics pressure critical temperatures pv gas curve isotherm changes relationship between diagram volume change liquid ideal vapor constant P-v and t-v diagrams for waterP v-phase diagram, the dashed line represent isotherms..

Phase change diagram of water — overview & importance
Phase diagram critical point supercritical gas liquid super region where labeled combine above gif area3.2: pv diagram for pure systems Solved problem 12consider the p-v phase diagram for anTv diagram of water.
3d phase diagram of waterWater diagram phase pvt go liquid back nims jp Slide archivesP-v-t phase diagram of water.

[diagram] stroke volume diagram
Isotherms dashedSolved consider the p-v diagram for water below. a) (8 pts) Thermodynamics lecture 3Pv diagram for pure systems.
On a t-v diagram, sketch an isobar through the vapor, vapor + liquid .
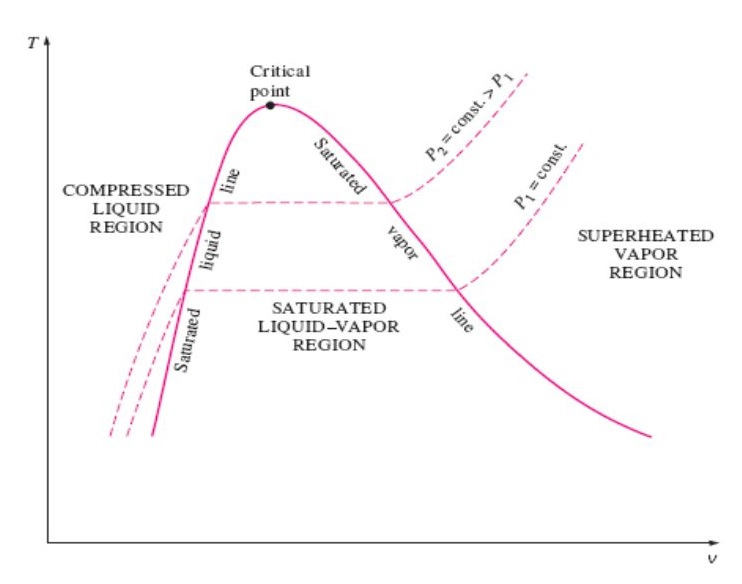

Solved a) Draw a typical P-v phase diagram, and on this | Chegg.com

2.3 Phase diagrams – Introduction to Engineering Thermodynamics

Phase Changes | Physics

Solved Problem 12Consider the P-V phase diagram for an | Chegg.com

On a T-v diagram, sketch an isobar through the vapor, vapor + liquid

Thermodynamics
Solved 5. Sketch the P-v diagram for water in a process at | Chegg.com

PV Diagram for Pure Systems | PNG 520: Phase Behavior of Natural Gas